
By Karis Chitty
As countries in Europe pause vaccinations of the AstraZeneca-Oxford vaccine due to safety concerns, Canada announced today that it has “expanded” its support for the shot.
But the bad press the vaccine is receiving could have an effect on Canadians’ feelings about the safety of vaccines.
That concerns one vaccine expert who is urging people who are worried to talk to their doctors before turning down the opportunity to get immunized.
“If they’re worried about the vaccines, they should talk to their physicians and depend on their advice, but these vaccines have been tested far beyond what is normally done,” says Jamie Scott, a professor emeritus in molecular biology and biochemistry (MBB) and founding member of SFU’s Faculty of Health Sciences who has spent decades specializing in vaccine-related research.
She believes the various European countries who have paused AstraZeneca-Oxford vaccinations due to reports of blood clot side effects are acting “overly cautious”.
“If you had a low rate of infection, you could afford that level of caution,” but not in this case, she says. “I think they’re being overly cautious and it’s at the expense of people dying of COVID…you’re potentially letting people die for very little reason,” Scott says.
In Canada, The National Advisory Committee on Immunization announced today that it has “expanded its recommendation for the use of the AstraZeneca vaccine,” saying that real-world effectiveness studies have shown it to be safe for people over 65 years.
And with COVID-19 cases and new variants still spreading everywhere, the World Health Organization cautioned that waiting longer for vaccines will have a much more dangerous outcome.
“As of today, there is no evidence that the incidences are caused by the vaccine, and it is important that vaccination campaigns continue so that we can save lives and stem severe disease from the virus,” WHO spokesman Christian Lindmeier said.
AstraZeneca and many other health experts joined in affirming WHO’s message, repeating that the number of cases of blood clots reported is actually lower than would be expected in a sample population of that size. This indicates no increased risk due to vaccination.
In spite of all this, the number of European countries putting the shot on pause continues to grow.
The bad press appears to have already increased vaccine hesitancy, particularly with this specific vaccine. Many European citizens share the idea that they would rather wait for a different vaccine than take the AstraZeneca-Oxford shot, as evidenced by the many available doses that have not been given out. Statistics suggest Canada may see the same results.
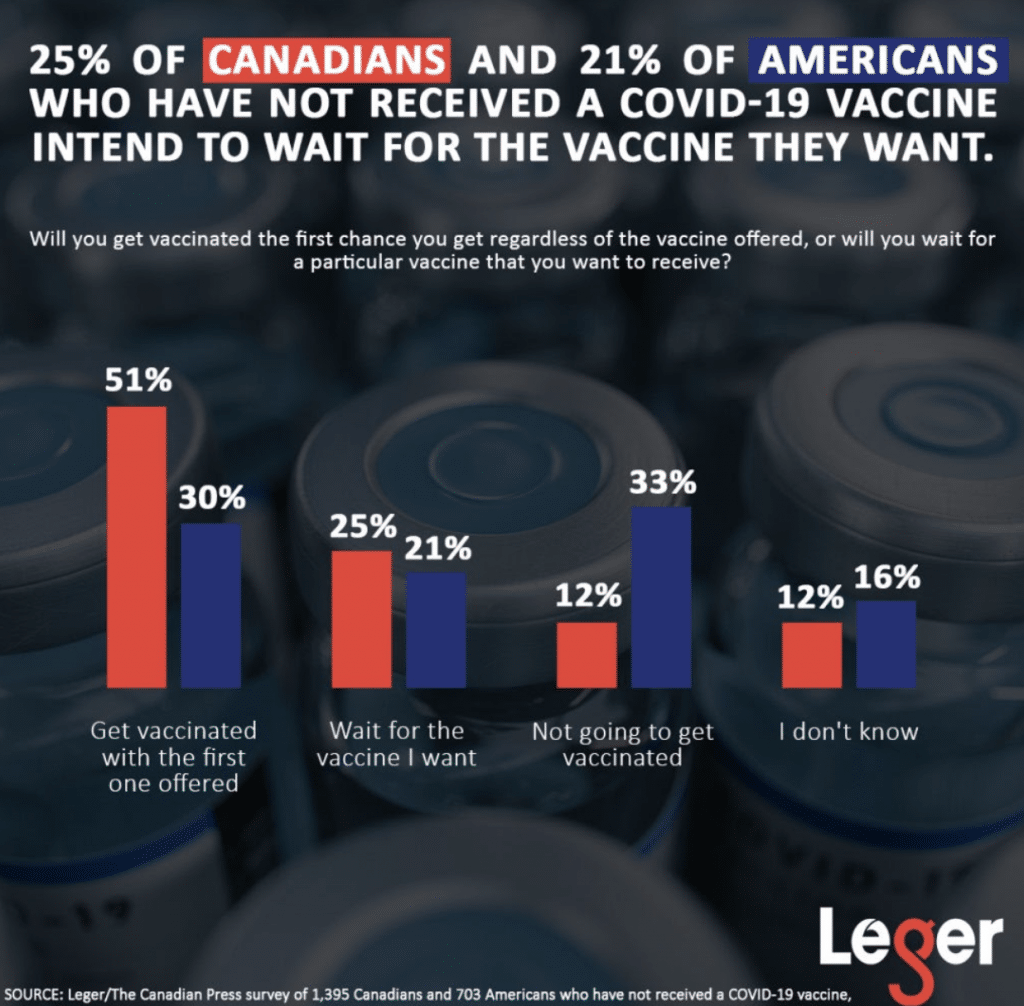
Last weekend, an online survey by Leger and the Association for Canadian Studies reported that 51 per cent of Canadians will take any of the four COVID-19 vaccines approved in Canada, while 25 per cent say they would rather wait to get the shot they prefer. We have yet to see if these statistics will change as Canadians react to the AstraZeneca-Oxford controversy.
Vaccine safety experts from the World Health Organization are meeting today to further discuss the situation.
